Alzheimer’s ‘step forward’ stalls: why a worthy-hyped drug goes through approval delays – Guardian

It became heralded in files articles as a “step forward”, a “turning point” and a “gamechanger” for Alzheimer’s disease. Some experts went to this point as to name the drug, donanemab, the “starting up of the stop” for the debilitating situation.
Pharmaceutical company Eli Lilly in Might well perhaps neutral 2023 launched files from a scientific trial they talked about confirmed donanemab slowed cognitive and purposeful decline in folks with early symptomatic Alzheimer’s disease by 35% over 18 months.
The findings noticed the head of Alzheimer’s Study UK and varied experts name on remedy regulators to suddenly approve the therapy to be used in patients.
Nonetheless despite stories the US remedy regulator became region to approve donanemab “any day”, the Food and Drug Administration (FDA) as a replacement presented on 8 March that it had delayed its resolution.
The FDA talked about it wants an neutral panel to extra scrutinise files on the protection and efficacy of donanemab, with a resolution now anticipated later in 2024. UK, European and Australian regulators are additionally aloof assessing the drug.
In a press free up, the govt. vice-president of Eli Lilly, Anne White, talked about: “We are confident in donanemab’s doubtless to present very meaningful advantages to folks with early symptomatic Alzheimer’s disease”.
“It became unexpected to be taught the FDA will convene an advisory committee at this stage within the overview route of, nonetheless we watch for the chance to extra contemporary the [trial] results and put donanemab’s rep efficacy within the context of safety,” she talked about. “We are in a position to work with the FDA and the stakeholders within the team to get that presentation and acknowledge all questions.”
Dr Timothy Daly, a dementia researcher with Sorbonne University in Paris, says this extend comes as no surprise to him.
He says the advantages of donanemab, and identical, worthy-hyped remedy, including aducanumab and lecanemab, maintain proved more durable to quantify than their doubtless harms.
“Under this chronicle of drug success, there are some in reality rep side-effects,” Daly informed Guardian Australia.
These are a form of drug identified as new monoclonal antibodies, and they aim amyloid proteins within the mind. Many researchers mediate the buildup of these proteins contributes to Alzheimer’s disease.

The remedy maintain been proven to prick amyloid phases within the mind. Nonetheless around three-in-10 folks taking lecanemab or donanemab in scientific trials developed a situation identified as amyloid-linked imaging abnormalities, abbreviated to ARIA, a situation which is in a situation to trigger mind swelling or haemorrhaging.
“Mostly these seem to be minor, not come with any indicators, and note-up scans display they seem to maintain resolved,” Dr Sebastian Walsh, a public properly being doctor researching dementia possibility low cost with the University of Cambridge within the UK, says.
“In a runt share of members it does seem to be worthy more serious, and there maintain been some deaths – namely for these on blood-thinning-form medicines.”
Some trial members additionally skilled mind shrinkage – and the prolonged-term effects of which are unknown.
‘It’s pure hypothesis’
In the donanemab trial, patients receiving the drug declined on common by 10 factors on a 144-point scale that mixed cognitive and purposeful rankings. The placebo team who weren’t receiving the drug declined by 13 factors.
This files became fashioned by researchers to dispute that the drug slowed cognitive and purposeful decline by “more than one-third”, and equipped folks “extra months” or “as much as twelve months of life” without extra disease progression.
Walsh says efforts to translate scientific files into phrases more meaningful for folks to fancy means the results of the drug maintain been overblown in media stories.
“While it’s a long way comprehensible that folks are making an attempt to mediate about varied programs to contemporary these numbers, it aloof needs to be scientifically staunch,” he says.
“Those that maintain reported it being ‘an extra six months at elevated feature’ are on shaky ground scientifically I mediate. The pains didn’t measure recognition of a liked one, ability to power, any of these items – extrapolating in this form will not be in reality justified by the evidence we now maintain got. It’s pure hypothesis.”
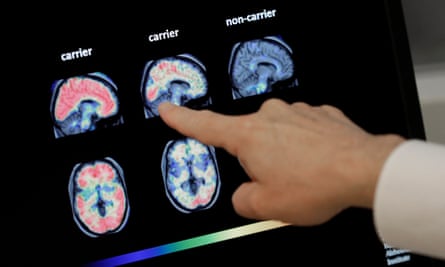
A professor of neurology at Radboud University Scientific Centre within the Netherlands, Edo Richard, informed files channel Al Jazeera the remedy “clearly take away” amyloid proteins from the mind “very successfully”.
Nonetheless a low cost in amyloid proteins does not basically consequence in a slowing of cognitive decline, he talked about.
after newsletter promotion
Study into the disease dating support more than 25 years has chanced on that amyloid proteins are contemporary within the brains of oldsters with dementia. Nonetheless in addition they’re chanced on in folks that don’t maintain dementia, and who never traipse on to make it, Richard informed Al Jazeera.
While many remedy trialled within the previous maintain diminished amyloid phases, donanemab, aducanumab and lecanemab seem like the first to maintain additionally ended in a switch in cognitive decline. Nonetheless Richard claimed that switch became “statistically predominant, nonetheless clinically beside the point”.
When the FDA authorized aducanumab in 2021, three FDA advisory committee members who suggested against its approval on account of what they believed became an absence of efficacy files resigned. One of many folks that resigned described it as “perhaps the worst drug approval resolution in most up-to-date US history”.
When it came to implementation, the US properly being insurance program Medicare talked about it wouldn’t duvet it, and clinicians maintain additionally been cautious, with little use of the drug.
The Australian regulator, the Therapeutic Goods Administration, in June chanced on “there may perhaps be not any such thing as a evidence of clinically meaningful efficacy” of aducanumab.
A ‘collective desperation’
As properly as minimal meaningful scientific advantages from donanemab, patients additionally must receive the remedy through an intravenous infusion at a scientific sanatorium or hospital as soon as every two to four weeks at a designate of about US$26,500, or A$40,500, a year plus undergo popular testing. It is loads to demand of vulnerable folks and their families.
Those that participate in scientific trials are additionally a highly selective team. In the donanemab trial, 1,320 members with amyloid and early disease indicators achieved it. For every 10 folks screened for eligibility for the trials, about eight were chanced on to be ineligible.
In a commentary written for the Conversation, Walsh talked about if, when prescribed within the true world, “the drug eligibility is limited to compare the trial eligibility, then very few folks will be eligible. If eligibility is broader, then already runt effects are inclined to be even smaller and side-effects more pronounced”.
The director of internal remedy and scientific epidemiology on the Princess Alexandra hospital in Queensland, Australia, Prof Ian Scott, printed a paper within the February version of the journal Age and Getting older with identical concerns. He wrote trials of amyloid-focusing on monoclonal antibodies to this point “attain not provide high-quality evidence of clinically meaningful impacts at an much less expensive designate”.
Daly believes that predominant focal point on the functionality of substances that target amyloid buildup despite an absence of efficacy has been reductive, because it has viewed much less attention being paid to quite loads of hypotheses of what is causing the disease, and programs to tackle it.
A 2020 picture from the Lancet commission on dementia estimated 40% of conditions of age-linked dementia are linked to 12 potentially modifiable possibility factors across the lifetime, including air pollution, obesity, depression, and much less training.
Daly says whereas such findings get it tempting to checklist standard of living changes folks can get to prick dementia possibility, this is additionally too simplistic, because it puts the onus on americans as a substitute of governments.
“Working conditions, sorts of oppression and things that can’t as without predicament be viewed as a dementia possibility are perfect as valuable in stopping disease,” Daly says.
“There may perhaps be an iceberg right here – don’t perfect peer on the bottom at remedy and standard of living. There are residing conditions and social structures that symbolize deeper contributions to possibility within the population, and interventions focusing on these are wanted by governments to get our society fairer and more dementia-resilient.”
Walsh says there may perhaps be understandably “a collective desperation” among scientists and patients for greater treatments and preventive alternate suggestions for Alzheimer’s disease, which is the most fashioned cause slack dementia in western societies and which has no remedy.
“Nonetheless this can not cloud objectiveness when we peer on the evidence,” he says.


